How does asbestos inhalation lead to cancer? Small mineral growths on asbestos fibres in the lungs may provide the answer.
Abstract
Inhalation of asbestos fibres is widely known to be associated with an aggressive type of cancer, called malignant mesothelioma (MM). Many industries utilised asbestos fibres in the past due to their incombustibility, heat resistance and flexibility. As such, many individuals from both industrial and domestic settings were exposed to tiny asbestos fibres. Investigations of the cause of the cancer have identified asbestos ferruginous bodies (AFBs), iron-rich mineral growths surrounding asbestos fibres, as a potential factor. So far this has been explained through the generation of reactive oxygen species (ROS), which form due to the interaction of lung fluids and the iron in the AFBs. The ROS produced may damage cells and their DNA and thus lead to the onset of MM. In this study we explore the possible ways this could happen through examining the internal morphology of AFBs for the first time. We also investigate whether there is any difference between the AFBs of smokers and non-smokers. Our results question whether ROS production is the main mechanism for MM onset and indicate that smoking may have some, as yet undetermined effect on MM onset/progression.
Paper
Maya-Liliana Avramescu, Christian Potiszil, Tak Kunihiro, Kazunori Okabe, and Eizo Nakamura, An investigation of the internal morphology of asbestos ferruginous bodies: Constraining their role in the onset of malignant mesothelioma, Particle and Fibre Toxicology, 20, 19 (2023), doi:10.1186/s12989-023-00522-0.
Short summary
- Malignant mesothelioma (MM) is a cancer of the mesothelium which is exclusively caused by a prolonged exposure to asbestos. Once the asbestos fibres are inhaled into the lung environment, the fibres accrete Fe in the form of ferrihydrite, which is contained within Fe transport proteins referred to as ferritin. The Fe rich coating and the asbestos core are referred to as asbestos ferruginous bodies (AFB).
- Whilst several studies have investigated the external morphology of AFBs, none have characterised the internal morphology. The internal morphology and chemical composition of AFBs from two smoker and two non-smoker MM patients were analysed using transmission electron microscopy (TEM), energy dispersive x-ray spectroscopy and selected area diffraction.
- The analysis revealed morphological and chemical differences between the AFBs of smokers and non-smokers which might be a result of different growth conditions in the lung environment. The AFBs of smokers were denser, smaller and more Fe-rich than the AFBs of non-smokers. The AFBs of smokers had a more uniform Fe supply in the lung environment than non-smokers likely due to Fe complexation of the cigarette smoke.
- The Fe phase in all analysed AFBs was of ferrihydrite, the most unstable and least crystalline of the iron oxyhydroxides, suggesting an intact ferritin shell and limited reaction with the pleural fluid. Thus, here it is suggested that the reactive oxygen species (ROS) may not be the main driver of MM.
Summary
Inhalation of asbestos fibres is widely known to be associated with an aggressive type of cancer, called malignant mesothelioma (MM). Many industries utilised asbestos fibres in the past due to their incombustibility, heat resistance and flexibility. As such, many individuals from both industrial and domestic settings were exposed to tiny asbestos fibres. Where the volume of fibres inhaled was very large, the upper respiratory track became overwhelmed and some portion of the fibres found their way into the lungs. Once in the lungs, the mechanism by which cancer develops and spreads to the mesothelioma is not well understood. So far theories have focussed on accepted cancer initiation mechanisms, such as reactive oxygen species (ROS). The ROS damage cells and attack biomolecules, including DNA, which can lead to mutations and eventually cancer. The production of ROS has been proposed to occur due to the iron (Fe) rich mineral growths on the asbestos fibres, called asbestos ferruginous bodies (AFBs), which can catalyse the formation of ROS through the Fenton reaction. However, this requires that the Fe is exposed and available to catalyse reactions. The likely source of the Fe is from ferritin, an Fe transport protein housed within macrophages that aim to prevent ROS formation through scavenging and isolating Fe from the body as a mineral called ferrihydrite. A previous study of the external morphology of AFBs found the presence of ferrihydrite and other Fe minerals, which indicates the breakdown of the ferrihydrite and confirmed the exposure of the iron. Nevertheless, this study was only conducted on the external morphology of the AFBs and it remains unclear how much ferrihydrite has been exposed to the lung environment. Here the internal morphology of the AFBs was examined using geochemical techniques and no other phases except ferrihydrite were observed. This finding raises doubts over how efficiently ROS could be produced from the AFBs and whether this can explain the onset of MM. Instead, high levels of the radioactive element radium (Ra) were found in the bulk measurements of AFBs previously (Nakamura et al., 2009) and thus mutations induced by DNA damage from ionising radiation may instead be more likely. Furthermore, differences were observed between the AFBs of smokers and non-smokers. Smokers were found to have smaller and denser AFBs than non-smokers, which was explained by a higher and more constant availability of Fe due to the Fe-complexation properties of cigarette smoke. Interestingly, the high Fe content of the AFBs may what allows them to adsorb and trap Ra. While no studies have found a difference between the mortality rate from smokers and non-smokers concerning MM, cigarette smoke was found to provide a synergistic effect for the onset of lung cancer in individuals who had inhaled asbestos fibres. Therefore, there may be an as yet undiscovered relationship between cigarette smoke and MM.
Full Text
Asbestos consists of fine fibres of silicate minerals and possess certain very useful properties, including incombustibility, heat resistance and flexibility. Such properties made asbestos a particularly inexpensive way of insulating buildings and ships and had the added benefit of not being a fire hazard. Accordingly, asbestos was seen as a safer alternative to other insulation materials. As a result of the widespread use of asbestos many individuals were exposed to it in both industrial and domestic settings. However, by the 1990’s the association between asbestos and malignant mesothelioma (MM), an aggressive cancer, became well established and lead to its widespread ban.
While asbestos is now not widely used, the latency period of asbestos induced MM is very long (15-50 years) and thus many individuals exposed to asbestos prior to the ban are expected to develop this cancer in the near future. As such, it is very important to better understand this disease, so that new treatments can be developed that can either extend the life expectancies of those contracting MM or help to cure it.
Currently, it is known that on exposure to large volumes of asbestos, the upper respiratory track becomes overwhelmed, and this prevents the body from removing the fibres via mucus transport and coughing. As a result, the fibres are able to penetrate deep into the lungs and damage the cells lining the lungs. Once in the lungs the fibres accumulate an iron (Fe)-rich coating, consisting of ferrihydrite, an Fe-rich mineral that is a major component of the Fe transport protein ferritin. Together the Fe-rich coating and the asbestos fibre are termed an asbestos ferruginous body (AFB, Figure 1).
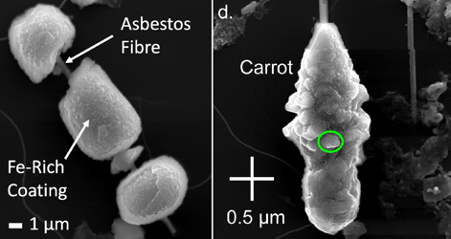
The presence of AFBs is closely associated with the development of MM and so it has been hypothesised that they may have some role in the onset of MM. At present, the most widely accepted theory is that the Fe-rich coating of the AFB generates reactive oxygen species (ROS) through a catalytic process termed the Fenton reaction. The ROS then damage cells and their DNA, which then leads to genetic mutations, which can in turn lead to the development of cancer cells. This line of reasoning was subsequently supported by the investigation of the external morphology of AFBs, which demonstrated the presence of both ferrihydrite and other Fe-rich phases. The presence of these other Fe-rich phases, suggested that the ferritin protein was damaged and the shell containing the Ferrihydrite core was exposed to the lung environment, where it was able to catalyse ROS production.
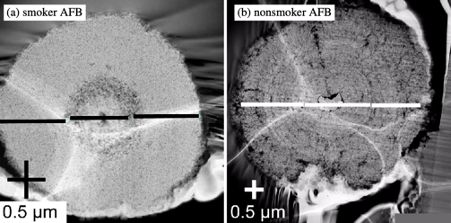
However, no internal morphological and chemical investigation of AFBs has been performed with high resolution instruments, such as transmission electron microscopy (TEM) in conjunction with energy dispersive X-ray spectroscopy (EDS). As such, it is not clear how AFBs grow over time or whether the presence of Fe-rich phases other than ferrihydrite are common to AFBs and found throughout their structure. Accordingly, here a TEM-EDS study was performed in order to better understand the formation of AFBs and their potential cancer-causing properties. In addition, selected area X-ray diffraction (SAED) was also performed to determine the crystal structure of the Fe-rich phases within the AFBs.
The results of the study indicated that the AFBs were composed of tiny sphere like materials that were rich in Fe (Figure 2). These spheres were found to be similar in size to rounded Fe-rich inclusions within macrophages reported from a study that injected asbestos fibre into the perinatal cavity of mice. The body's immune response to foreign objects is to try and engulf them and break them down with certain chemicals, such as enzymes. Macrophages are the white blood cells responsible for this process, but they cannot break down the asbestos fibres and are not big enough to fully engulf them. This leads to a process known as frustrated phagocytosis, in which the macrophages die on the fibre. If the macrophages have recently engulfed iron rich material, such as dead red blood cells or iron rich dust particles, then this can be housed within the macrophages as ferrihydrite contained with ferritin and may be the iron rich sphere reported previously. Therefore, AFBs may grow as a result of the accumulation of ferritin spheres from dead macrophages (Figure 3).
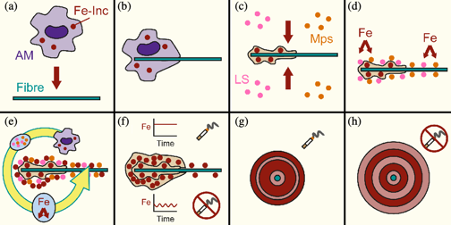
On investigating the minerals, the SAED patterns indicated that all phases were some form of ferrihydrite. This brought into question the notion that the Fe-rich coating of the AFBs could be producing ROS, because the Ferrihydrite may have remained encased within its protein shell. If the ferrihydrite was not exposed to the lung environment, how could it catalyse ROS production?
An alternative theory may be the presence radium (Ra), a radioactive element, in AFBs. Radium was found to be present at heightened levels in AFBs from a previous bulk chemistry study and Fe-rich materials are particularly could at adsorbing Ra. Radioactive materials, like radium, decay and release ionising radiation, which like ROS can damage cells and DNA. Therefore, the AFBs may act like a sponge for radioactive elements present in the atmosphere or ground water and their radiation may be the cause of MM. While future work is required to establish this theory more thoroughly, the present study has highlighted the need to look for such alternative theories and has suggested a suitable hypothesis to test.
Another key finding of the current study was the discovery that smokers and non-smoker had differences in terms of their AFB morphology. The smokers had smaller and more dense AFBs, than the non-smokers. A possible explanation for this is that cigarette smoke can complex Fe in the lung environment, such as within lung fluids, and provide a steady source of Fe. For non-smokers the Fe availability likely fluctuates and this leads to larger more porous AFBs (Figure 3).
Such a finding is interesting, because while no study has found that smoking can influence the onset of asbestos induced MM, studies have found that smoking has a synergistic effect on lung cancer formation in patients who have inhaled asbestos. Therefore, it may be the case that smoking affects the onset or progression of mesothelioma in some as yet unknown way and this should be investigated further.
In summary, the present study investigated the internal morphology and chemistry of AFBs and used this information to assess the currently widely accepted ROS model for MM cancer onset and progression. The results have brough into question such a model, as no evidence for the alteration of ferrihydrite to other Fe-phases was observed, indicating that ferrihydrite may be still encased within its ferritin protein host. As such an alternative theory involving ionising radiation from Ra was proposed. Furthermore, different AFB morphologies between smokers and non-smoker highlights the potential for smoking to have some effect on the onset of MM or its progression.