Discussing Science: Comment on: ‘A simple cryogenic method for efficient measurement of triple oxygen isotopes in silicates’ by Ghoshmaulik et al.
09/04/2020
In a recent article by Ghoshmaulik et al. at Rapid Communications in Mass Spectrometry, described technical modifications to a long-established laser-assisted fluorination procedure for extracting and purifying molecular oxygen from silicates, prior to triple-isotope ratio analysis. Although the reported respective measurement precisions for δ17O and δ18O values, at 0.040‰ and 0.080‰, are identical to those reported more than twenty years ago, using a similar (but not identical) system and protocol, the very tight coupling of δ17O and δ18O measurement errors in the new method permits the determination of Δ′17O values to a precision of 4 ppm, or even less. All precision values discussed herein refer to one standard deviation (σ). The description of how Ghoshmaulik et al achieved such precise Δ′17O values is a welcome contribution to the increasing number of reports quantifying small but distinctive variations between the relative abundances of 17O and 18O in terrestrial silicate rocks and minerals.
Distinct from Δ′17O precision – which is independent of calibration of the δ17O and δ18O data to any particular reference material – is the corresponding accuracy of the data on a designated scale. Conversion of the empirical δ17O and δ18O data, as reported relative to a ‘working standard’ O2, to the corresponding values relative to the Vienna Standard Mean Ocean Water (VSMOW) reference, with a degree of accuracy commensurate with the Δ′17O precision, is challenging. The difficulty is, as least in part, compounded by the use of a water reference for the δ17O and δ18O scales, whereas silicates and waters require different fluorination procedures for the extraction of molecular oxygen, the analyte gas used for the triple-isotope ratio measurements. Furthermore, few laboratories have the capability to make such measurements on silicates and on waters. Even those that do, which (in principle, at least) enables accurate calibration of the ‘working standard’ O2 to VSMOW, and any instrument-related compression of the δ17O and δ18O scales to be quantified from measurements of the Standard Light Antarctic Precipitation (SLAP) reference, for which Δ′17O is defined to be zero for the VSMOW-SLAP scale, may nevertheless report differing Δ′17O values for commonly-used silicate standards such as San Carlos olivine, UWG-2 garnet and NBS 28 quartz. This is despite using the same definition for Δ′17O.
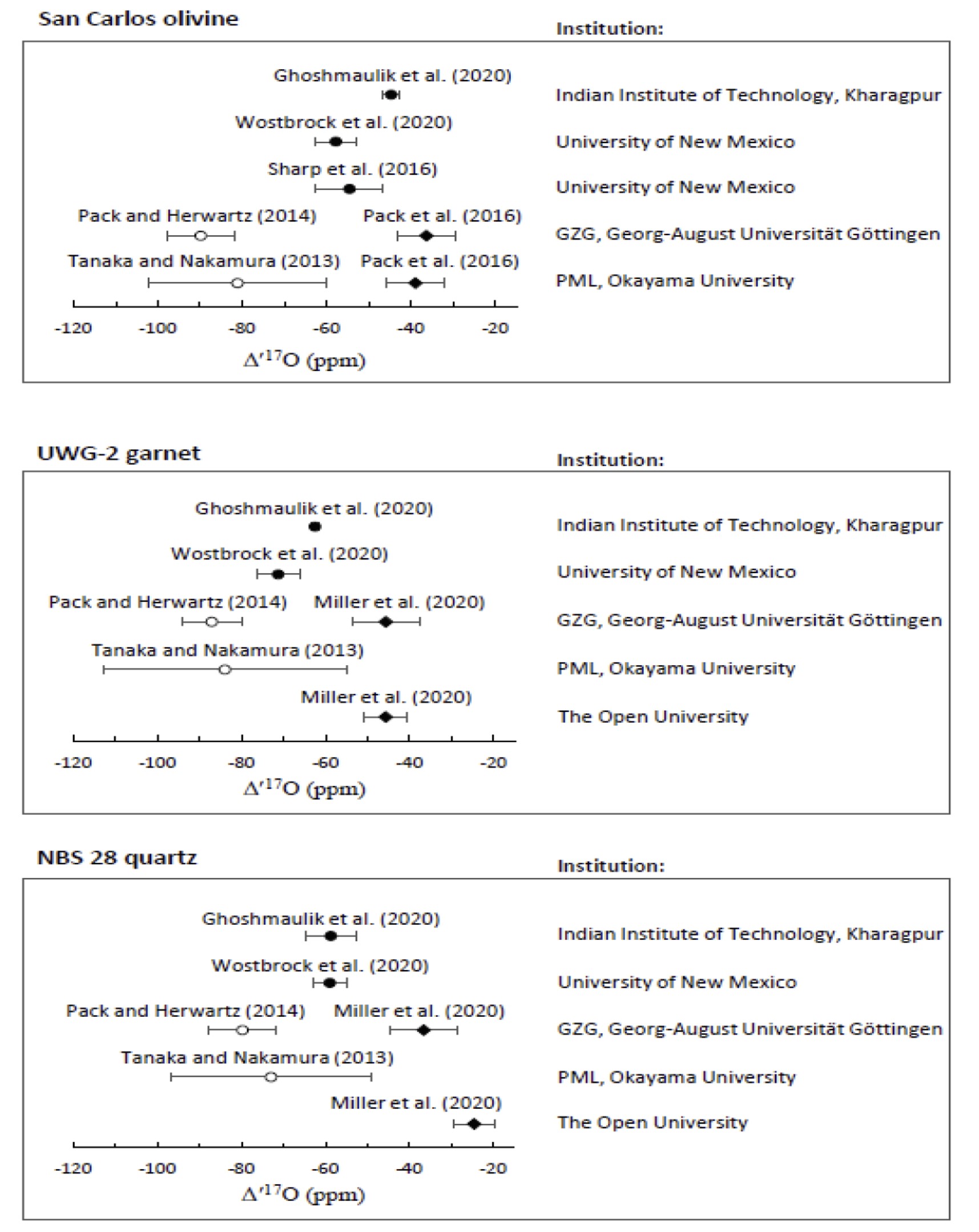
Ghoshmaulik et al suggested that their cryogenic purification protocol efficiently removes NF3 contaminants from oxygen gas produced by laser fluorination of silicates, without the need to use gas chromatography for isolating the oxygen from other component that may be present. In a two previous inter-laboratory investigations, however, gas chromatographic purification of the oxygen was used at one of the institutions, but not at the other. Yet very good agreement was obtained for the Δ′17O value of San Carlos olivine in the one study that measured only that silicate standard, and for three of the four silicates (all except NBS 28) investigated in the other study. Even without implementing the procedural improvements suggested by Ghoshmaulik et al, therefore, a gas chromatographic purification step does not seem to be essential to obtaining Δ′17O values of high precision and reproducibility. Furthermore, we have not found NF3 to be detectable in the oxygen extracted from silicates such as San Carlos olivine, UWG-2 garnet and NBS 28 quartz. In our experience, it is usually when analyzing samples of specific types of meteorites (notably, carbonaceous chondrites) that NF3 is likely to be produced during the silicate fluorination step. The accuracy and precision of Δ′17O determinations are probably controlled principally by the efficiency of molecular oxygen adsorption and desorption to/from the cryo-cooled zeolite pellets, assuming that the oxygen ‘blank’ level associated with the complete extraction and purification procedures is shown to be of negligible magnitude and that the BrF5 used for the fluorination step is of high purity. Consequently, details of the cryogenic transfer arrangement and protocol are critical, as is the complete removal of volatile contaminants from the zeolite pellets between the analysis of successive samples. The procedure described by Ghoshmaulik et al is therefore a welcome contribution, which offers the potential for improving the precision of silicate Δ′17O determinations. The challenge remains, however, to understand why different laboratories which calibrate oxygen triple-isotope data directly to VSMOW or to the VSMOW-SLAP scale do not obtain consensus on the Δ′17O values of San Carlos olivine, UWG-2 garnet and NBS 28 quartz.
References:
- Matin Miller, Ryoji Tanaka, Richard Greenwood. Comment on: ‘A simple cryogenic method for efficient measurement of triple oxygen isotopes in silicates’ by Ghoshmaulik et al., Rapid Communications in Mass Spectrometry, (2020). doi:10.1002/rcm.8913.